Rapid, On-Site, Viral Vector-Free Car T-Cell Manufacturing Gives Faster Production of Higher Quality Cell Population (RFT-538)
Invention Summary
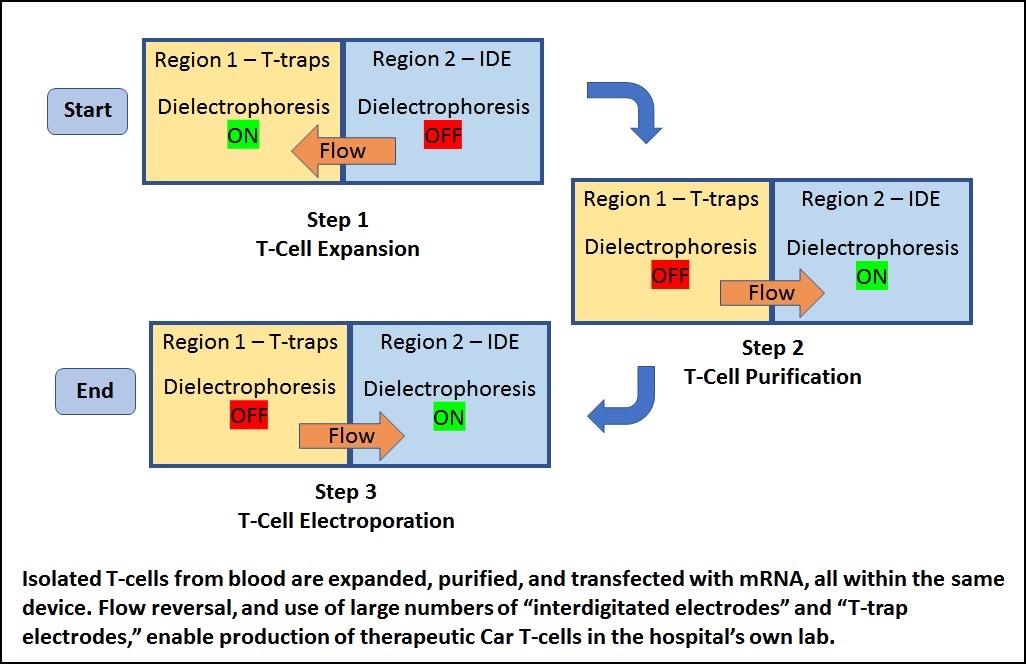
Scientists at NDSU have developed a small device for improved CAR T cell production, which speeds the turnaround time by enabling CAR T cell production 'on-site' at a hospital or cancer clinic. This simple, microfluidic device is easy to make and use, with an automated transfection process that takes about one hour. Because this process takes place at the clinic, a physician can a have another treatment ready when the preceding treatments winds down, and the physician can also adjust each treatment (number of CAR T cells and CAR molecules per cell) to optimize treatment while minimizing adverse effects. The NDSU device improves the quality and viability of the CAR T cell population. Quality is improved in part because this device uses radio frequency (RF) electric fields rather than DC electric fields. The RF electric fields drive mRNA into T cells, enabling more mRNA per cell (and resulting in potentially greater efficacy per CAR T cell). Additionally, the device uses an array of micro-electrodes that minimize exposure of cells to electric fields, and thereby increases cell viability. Initial estimates are that this technology results in up to 95% cell viability, and that the anti-cancer activity lasts about 2 weeks. Finally, this device provides a capability to remove dying T cells, thereby avoiding potential problems that come from having a large number of dying cells in the CAR T cell population. The NDSU device doesn't use viruses and there is no genome modification of T cells.
Benefits
- More rapid turnaround of therapeutic CAR T Cells than sending to specialized facility
- Allow physicians to personalize therapy to address an individual's needs (e.g. number of cells, expression levels of CAR molecules, and frequency of the dose)
- Allow physicians to rapidly adjust treatment parameters, e.g. in cases where patients have over- or under-reaction to the initial approach, thereby reducing the degree and duration of side-effects
- Uses RF electric fields, pushing more mRNA into each CAR T cell, while causing less cell damage (so that a significantly higher percentage of cells are active and viable)
- Capable of removing dying cells, so the population doesn't experience negative effects of a large number of dying cells, and has a longer period of anti-cancer activity in the patient
- Enables miniaturization and automation, and eliminates dependence on costly external laboratory services, substantially reducing the costs to provide CAR T therapy
Patents
This technology is patent pending in the US 20220266214A1 and is available for licensing/partnering opportunities.
Contact
NDSU Research Foundation
info(at)ndsurf(dot)org
(701)231-8173
NDSURF Tech Key
RFT, 538, RFT538
Inquire about this technology >